What's New?
Recent publications from our staff here at Proteoma
Recent Publications
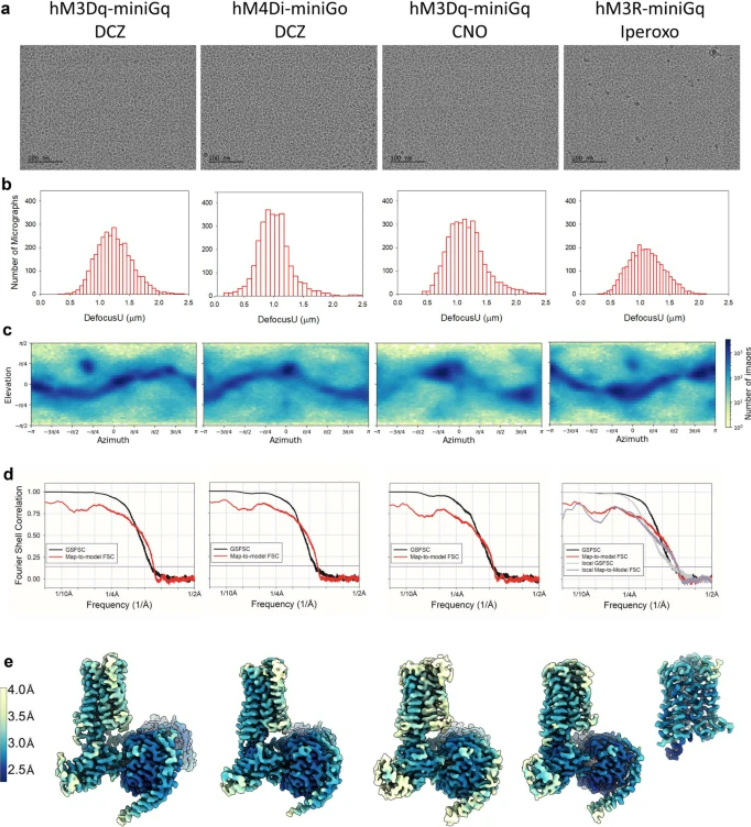
Molecular basis for selective activation of DREADD-based chemogenetics
Published in Nature, November 2022
Designer receptors exclusively activated by designer drugs (DREADDs) represent a powerful chemogenetic technology for the remote control of neuronal activity and cellular signalling1,2,3,4. The muscarinic receptor-based DREADDs are the most widely used chemogenetic tools in neuroscience research. The Gq-coupled DREADD (hM3Dq) is used to enhance neuronal activity, whereas the Gi/o-coupled DREADD (hM4Di) is utilized to inhibit neuronal activity5. Here we report four DREADD-related cryogenic electron microscopy high-resolution structures: a hM3Dq–miniGq complex and a hM4Di–miniGo complex bound to deschloroclozapine; a hM3Dq–miniGq complex bound to clozapine-N-oxide; and a hM3R–miniGq complex bound to iperoxo. Complemented with mutagenesis, functional and computational simulation data, our structures reveal key details of the recognition of DREADD chemogenetic actuators and the molecular basis for activation. These findings should accelerate the structure-guided discovery of next-generation chemogenetic tools. READ MORE
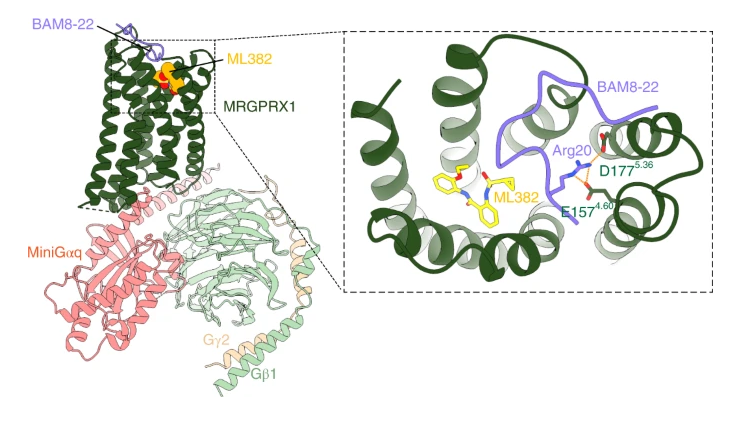
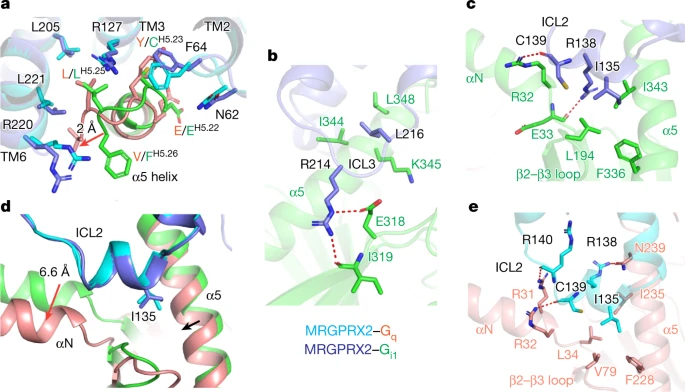
Ligand recognition and allosteric modulation of the human MRGPRX1 receptor
Published in Nature Chemical Biology, October 2022
The human MAS-related G protein–coupled receptor X1 (MRGPRX1) is preferentially expressed in the small-diameter primary sensory neurons and involved in the mediation of nociception and pruritus. Central activation of MRGPRX1 by the endogenous opioid peptide fragment BAM8-22 and its positive allosteric modulator ML382 has been shown to effectively inhibit persistent pain, making MRGPRX1 a promising target for non-opioid pain treatment. However, the activation mechanism of MRGPRX1 is still largely unknown. Here we report three high-resolution cryogenic electron microscopy structures of MRGPRX1–Gαq in complex with BAM8-22 alone, with BAM8-22 and ML382 simultaneously as well as with a synthetic agonist compound-16. These structures reveal the agonist binding mode for MRGPRX1 and illuminate the structural requirements for positive allosteric modulation. Collectively, our findings provide a molecular understanding of the activation and allosteric modulation of the MRGPRX1 receptor, which could facilitate the structure-based design of non-opioid pain-relieving drugs. READ MORE
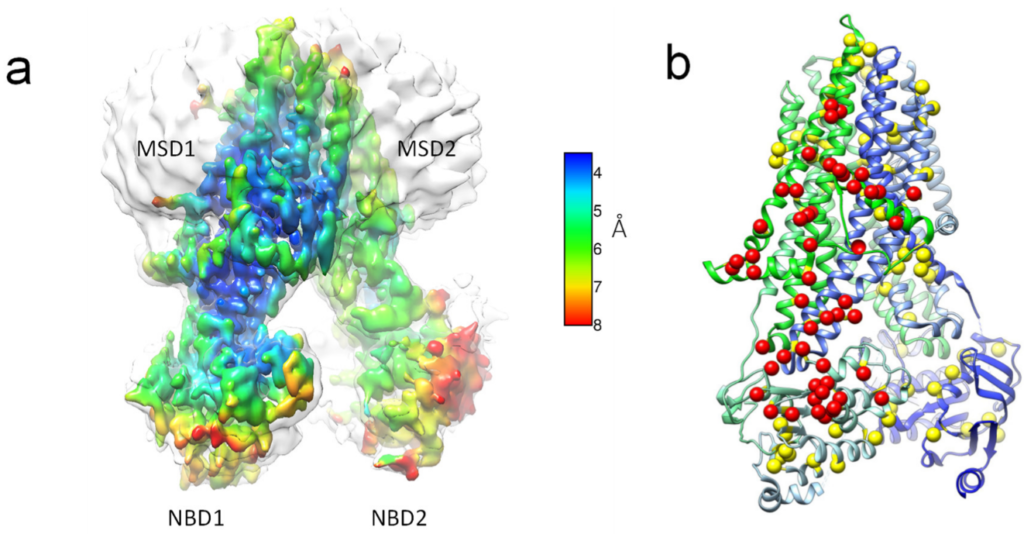
Conformational Variability in Ground-State CFTR Lipoprotein Particle Cryo-EM Ensembles
Published in International Journal of Molecular Sciences, August 2022
Cystic fibrosis transmembrane regulator (CFTR) is a dynamic membrane protein belonging to the ABC transporter family. It is unusual within this family as it is an ion channel, as opposed to a transporter. Activation of CFTR requires ATP and phosphorylation by PKA, and dysregulation of CFTR mediated salt and water homeostasis can lead to cystic fibrosis. Recent advancements in structural biological methods have led to more than 10 published CFTR structures, and, so far, all of these structures of CFTR, determined by cryo-EM, have been limited to detergent-purified protein preparations. To visualize CFTR in an environment that more closely represents its native membranous environment, we utilized two different lipoprotein particle encapsulation techniques: one in which the ion channel is first purified and then reconstituted using the membrane scaffolding protein Saposin A and another that uses the solubilizing polymer Sokalan CP9 (DIBMA) to extract CFTR directly from membranes. Structures derived from these types of preparations may better correlate to their function, for instance, the single-channel measurements from membrane vesicles. READ MORE
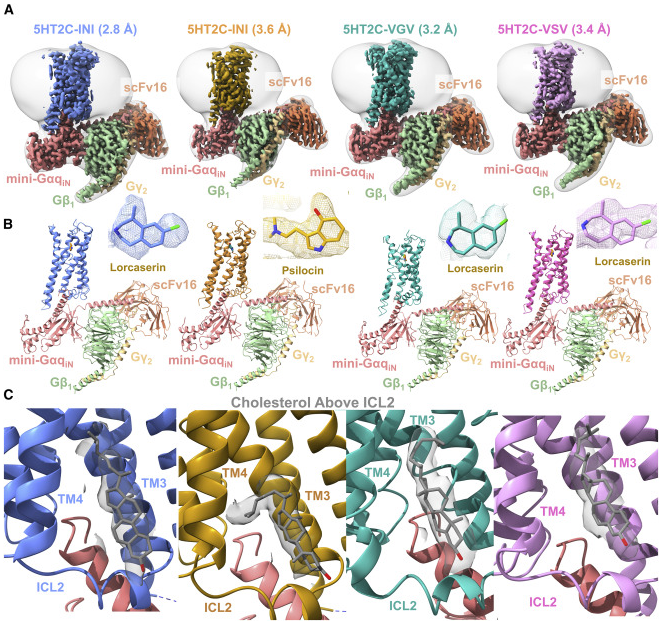
Published in Cell Reports, August 2022
RNA editing is a process by which post-transcriptional changes of mRNA nucleotides alter protein function through modification of the amino acid content. The 5HT2C serotonin receptor, which undergoes 32 distinct RNA-editing events leading to 24 protein isoforms, is a notable example of this process. These 5HT2C isoforms display differences in constitutive activity, agonist/inverse agonist potencies, and efficacies. To elucidate the molecular mechanisms responsible for these effects of RNA editing, we present four active-state 5HT2C-transducer-coupled structures of three representative isoforms (INI, VGV, and VSV) with the selective drug lorcaserin (Belviq) and the classic psychedelic psilocin. We also provide a comprehensive analysis of agonist activation and constitutive activity across all 24 protein isoforms. Collectively, these findings reveal a unique hydrogen-bonding network located on intracellular loop 2 that is subject to RNA editing, which differentially affects GPCR constitutive and agonist signaling activities. READ MORE
Published in Microscopy and Microanalysis, July 2022
Optimization of single-particle cryo-electron microscopy (cryo-EM) data collection routines is critical for ensuring efficient use for cryo-TEM beam time. Recently, we showed that it is possible to collect high-resolution cryo-EM data on a 200 keV Talos Arctica G3 cryo-TEM equipped with a Gatan K3 direct-electron detector at speeds of up to 720 movies per hour. EM maps of mouse apoferritin were collected with SerialEM using beam-image shift (BIS) data collection. Speed was dependent on multiple factors including the number of micrographs collected within each multishot (TEM grid hole size and spacing), the image-shift delay factor, and detector binning. We found data collection using super-resolution mode (SR) to proceed slower compared to hardware-binned mode (HWB). The resulting apoferritin maps reconstructed from data sets collected at a nominal magnification of 54,900X corresponding to a physical pixel size of 0.88A in either SR or HWB mode were comparable, with he estimated resolution ranging from 1.8-1.9A. READ MORE
Published in Nature Communications, July 2022
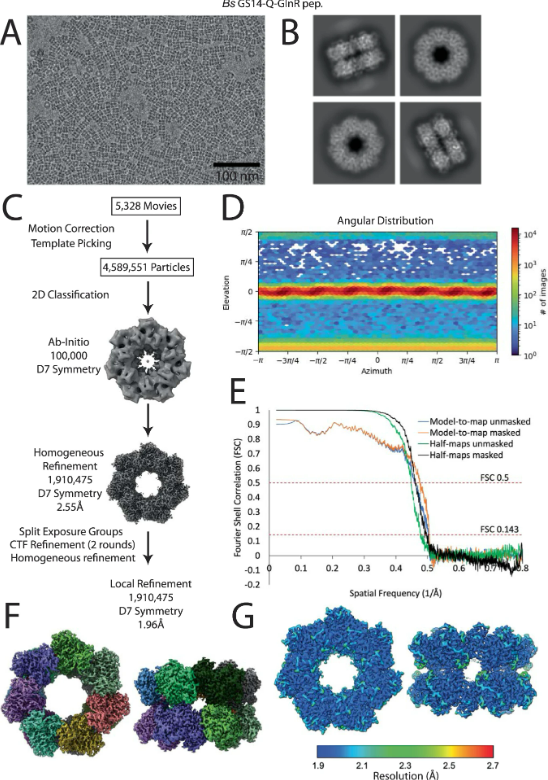
How bacteria sense and respond to nitrogen levels are central questions in microbial physiology. In Gram-positive bacteria, nitrogen homeostasis is controlled by an operon encoding glutamine synthetase (GS), a dodecameric machine that assimilates ammonium into glutamine, and the GlnR repressor. GlnR detects nitrogen excess indirectly by binding glutamine-feedback-inhibited-GS (FBI-GS), which activates its transcription-repression function. The molecular mechanisms behind this regulatory circuitry, however, are unknown. Here we describe biochemical and structural analyses of GS and FBI-GS-GlnR complexes from pathogenic and non-pathogenic Gram-positive bacteria. The structures show FBI-GS binds the GlnR C-terminal domain within its active-site cavity, juxtaposing two GlnR monomers to form a DNA-binding-competent GlnR dimer. The FBI-GS-GlnR interaction stabilizes the inactive GS conformation. Strikingly, this interaction also favors a remarkable dodecamer to tetradecamer transition in some GS, breaking the paradigm that all bacterial GS are dodecamers. These data thus unveil unique structural mechanisms of transcription and enzymatic regulation. READ MORE
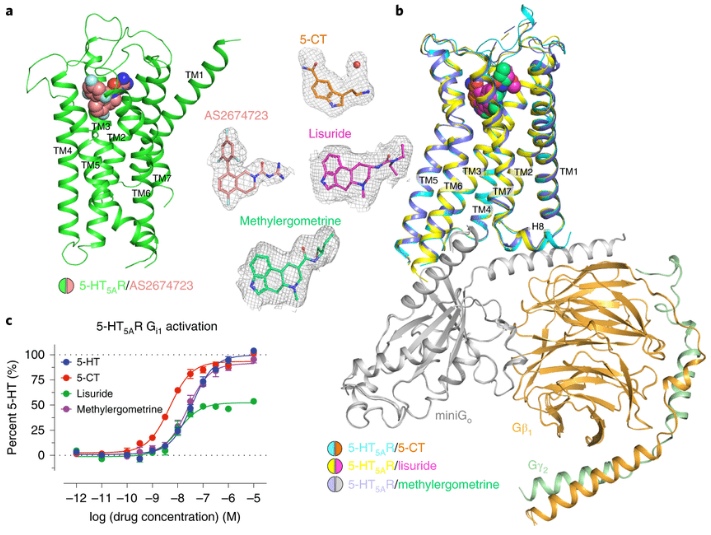
Inactive and active state structures template selective tools for the human 5-HT5A receptor
Published in Nature Structural & Molecular Biology, July 2022
Serotonin receptors are important targets for established therapeutics and drug development as they are expressed throughout the human body and play key roles in cell signaling. There are 12 serotonergic G protein-coupled receptor members encoded in the human genome, of which the 5-hydroxytryptamine (5-HT)5A receptor (5-HT5AR) is the least understood and lacks selective tool compounds. Here, we report four high-resolution (2.73–2.80 Å) structures of human 5-HT5ARs, including an inactive state structure bound to an antagonist AS2674723 by crystallization and active state structures bound to a partial agonist lisuride and two full agonists, 5-carboxamidotryptamine (5-CT) and methylergometrine, by cryo-EM. Leveraging the new structures, we developed a highly selective and potent antagonist for 5-HT5AR. Collectively, these findings both enhance our understanding of this enigmatic receptor and provide a roadmap for structure-based drug discovery for 5-HT5AR. READ MORE
High-speed high-resolution data collection on a 200 keV cryo-TEM
Published in International Union of Crystallography Journal, March 2022
Limitations to successful single-particle cryo-electron microscopy (cryo-EM) projects include stable sample generation, production of quality cryo-EM grids with randomly oriented particles embedded in thin vitreous ice and access to microscope time. To address the limitation of microscope time, methodologies to more efficiently collect data on a 200 keV Talos Arctica cryo-transmission electron microscope at speeds as fast as 720 movies per hour (∼17 000 per day) were tested. In this study, key parameters were explored to increase data collection speed including: (1) using the beam-image shift method to acquire multiple images per stage position, (2) employing UltrAufoil TEM grids with R0.6/1 hole spacing, (3) collecting hardware-binned data and (4) adjusting the image shift delay factor in SerialEM. Here, eight EM maps of mouse apoferritin at 1.8–1.9 Å resolution were obtained in the analysis with data collection times for each dataset ranging from 56 min to 2 h. An EM map of mouse apoferritin at 1.78 Å was obtained from an overnight data collection at a speed of 500 movies per hour and subgroup analysis performed, with no significant variation observed in data quality by image shift distance and image shift delay. The findings and operating procedures detailed herein allow for rapid turnover of single-particle cryo-EM structure determination. READ MORE
Structure, function and pharmacology of human itch GPCR’s
Published in Nature, November 2021
The sensation of itch (pruritis) can be triggered by many environmental insults, including insect bites, parasites, skin diseases such as eczema, liver and kidney diseases, and hypersensitivity reactions to commonly prescribed medications7. Itch has both neuronal7 and non-neuronal components, with histamine release from mast cells being prominent8. Several transmitters have been implicated in sensing and mediating the itch response, including histamine, interleukin and various peptides. Molecular targets involved in itch include G-protein-coupled receptors (GPCRs)…. READ MORE